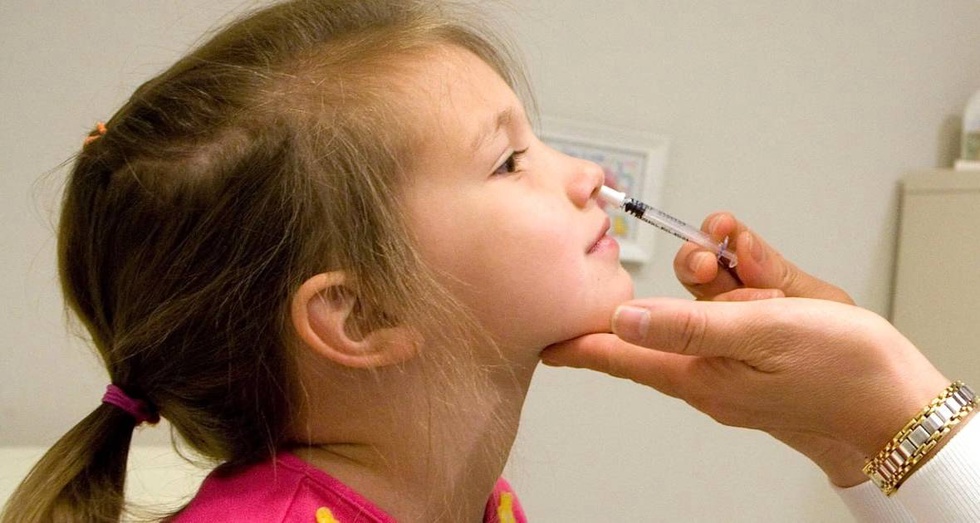
According to the FDA, FluMist is the first vaccine for the prevention of influenza, for the introduction of which you do not need to go to a medical institution.
AstraZeneca plans to sell the vaccine through a third-party online pharmacy. When ordering FluMist, customers will need to undergo screening to confirm the possibility of its use.
The vaccine was first approved in 2003, but only medical professionals could administer it. The vaccine contains weakened live strains of the influenza virus.