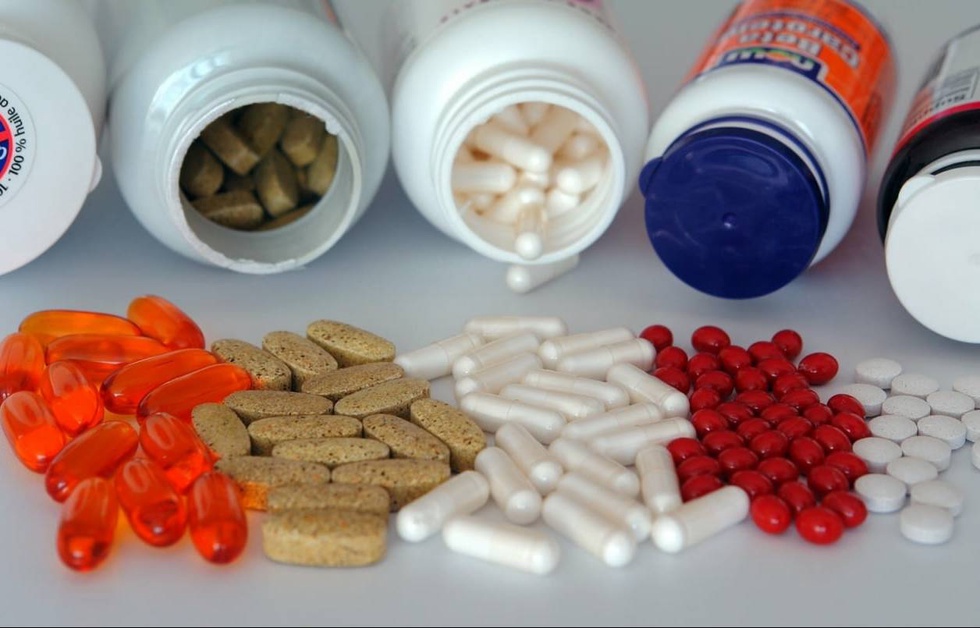
The inspection showed that the composition of dietary supplements KIFTI ORSA, ORO, OS MAX, BRINE and FATA BORSE turned out to be identical to the drug ORSA registered by Jurabek Laboratories. In addition, the instructions for the supplements contained inaccurate information about the properties of the drugs.
The Committee demanded to stop the production of these dietary supplements, to withdraw products already supplied from retail chains and to revoke the permits received by the companies for the production of additives.
The ministry noted that recently there have been a number of attempts to register medicines under the guise of dietary supplements. This allows manufacturers to circumvent the strict requirements and complex procedures required to enter the market for a new drug. The Committee promised to develop proposals to prevent such cases.