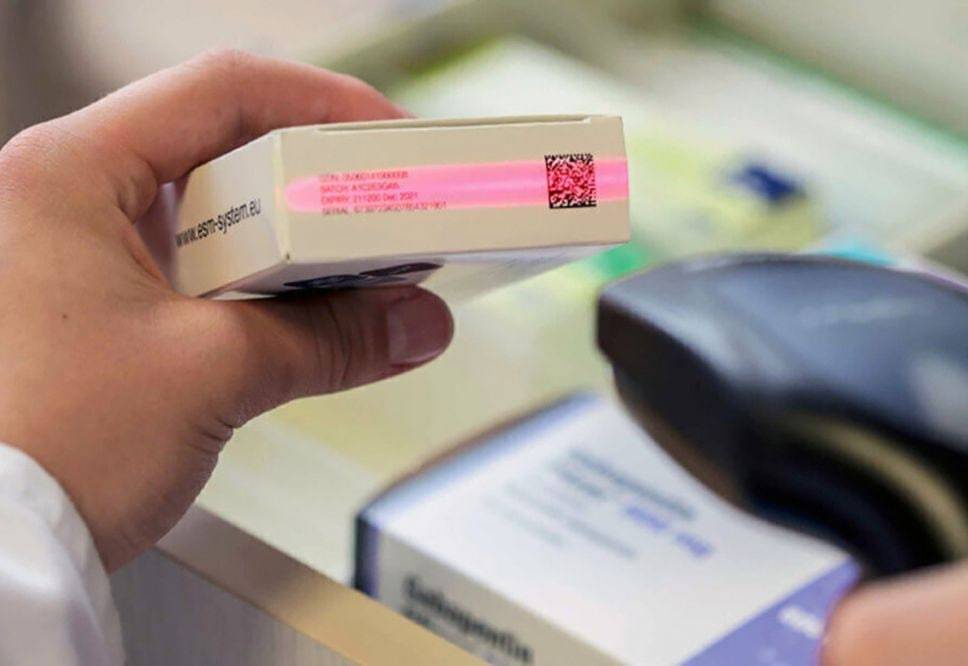
The document provides for the phased introduction in 2022-2025 of a system of mandatory digital labeling by means of identification, taking into account groups of medicines (except substances) and medical devices.
The following deadlines have been set for the start of mandatory digital labeling and types of products by groups:
- the first group - medicines in secondary (external) packaging (except orphan drugs) - from September 1, 2022;
- the second group - medicines in primary (internal) packaging and angro-drugs (except orphan) - from November 1, 2022;
- the third group - orphan drugs (for the treatment of rare diseases) - from March 1, 2023;
- the fourth group - imported medicines included in the special list - from February 1, 2025.
The resolution approved:
- list of identification codes of medicines and medical devices with mandatory digital labeling;
- schedule of phased implementation of the mandatory digital labeling system by domestic manufacturers of medicines and medical devices;
- schedule of phased implementation of the mandatory digital labeling system by foreign manufacturers of medicines and medical devices;
- schedule of gradual transition of wholesale and retail trade organizations to the system of mandatory digital labeling of medicines and medical devices;
- schedule of gradual transition of medical organizations in the field of healthcare to the system of mandatory digital labeling of medicines and medical devices;
- Regulation on the procedure for digital labeling of medicines and medical devices by means of identification.
The document was published in the National Database of Legislation and entered into force on 02.04.2022, the legal portal "Norma" reports.